Rapid Deployment Vaccine Collaborative (RaDVaC) Receives $2.5M from Balvi Filantropic Fund to Improve Global Vaccine Access
May 3, 2022
The Balvi grant will enable continued development, testing, and continuous updates and improvements of open-source vaccine designs and production methods, including “vaccine factory in a tube” technology. Funds will also support build-out of a decentralized scientific network for open sharing of production methods and technologies, as well as clinical trial and immunology data.
The Rapid Deployment Vaccine Collaborative (RaDVaC) is pleased to announce a $2.5M grant from Balvi, the philanthropic organization established by Ethereum co-founder Vitalik Buterin, which funds high-risk, high-reward projects to improve global pandemic preparedness and response capabilities.
The SARS-CoV-2 pandemic continues to reveal very serious, ongoing deficiencies in global pandemic readiness and public health responses. Once lifesaving vaccines were deployed, they showed very high levels of effectiveness. However, despite having been designed and produced in the earliest days of the pandemic, due to complex, slow, and inefficient regulatory burdens, they remained inaccessible for nearly a year in high-income countries, and for far longer in low- and middle-income countries. In response to this urgent humanitarian need caused by the vaccine access gap, RaDVaC developed and published free and open-source vaccine recipes and protocols, and offered scientific support to individuals and groups worldwide producing and testing the vaccines.
Since early 2020, RaDVaC has been creating technically accessible open-source vaccine designs and development tools, and constantly refining them in light of ongoing research. RaDVaC will use funds from Balvi to expand these activities and to create distributable vaccine resources, including “vaccine factory in a tube” technology.
RaDVaC and Balvi share a commitment to bridging and shortening the vaccine access gap, and in establishing both the citizen-science right to vaccine creation, and vaccine access as a public good. More broadly, both organizations share the goal of fostering decentralized, open-source research and knowledge dissemination.
With this grant, RaDVaC will be able to expand its efforts to enhance global pandemic preparedness on a number of fronts.
Year-End Review & Updates, 2021
Dear Radvackers,
As 2021 comes to a close, we see hopeful glimpses of a waning pandemic. Thus far, the omicron variant seems milder, and even though it is spreading rapidly and we are bracing for a surge into 2022, we are also hopeful that we will emerge with much higher immunity from combinations of vaccination and natural infection. Nevertheless, these latter developments cannot lull us into a false sense of future security. Omicron will not be the last SARS-CoV-2 variant, and a more dangerous but also highly transmissible variant might yet arise from the many remaining global reservoirs of virus. However quickly they emerge, future outbreaks and pandemics lie ahead, and are best dealt with preventively rather than reactively.
As we transition into the new year, newly hopeful but ever committed to building a better global health future, the core team at RaDVaC want to take this moment to express our deepest gratitude to the many who participated in the audacious global experiment called RaDVaC. Thanks to the many who tested and experimented with our vaccine designs. Thanks to those who shared vaccine design variations and efficacy data with us. Thanks to outspoken and public supporters of freedom of self-determination in the face of an invisible but deadly enemy. And thanks to those individuals and organizations who provided financial support to the RaDVaC project.
Many of our newest subscribers became aware of us in response to the news that RaDVaC is a recent recipient of an Astral Codex Ten grant, which we’re honored to receive. Scott Alexander’s endorsement means a lot to us, and the financial support will be put to good use. Read on for details about that.
All of your collective contributions have helped make 2021 a year of great progress. Here are some highlights of RaDVaC’s accomplishments in 2021:
- Better vaccines: Gen 10 and beyond. We made multiple, substantial improvements to the general vaccine platform, all of which are usable in vaccines against pathogens beyond SARS-CoV-2.
- Improved nanoparticle vehicle and adjuvant. The primary vaccine delivery vehicle and adjuvant chitosan proved suboptimal, due primarily to its insolubility in water; therefore, we investigated and experimented with some options and settled on the use of a chitosan derivative that goes by the acronym HACC. HACC has all of the benefits of chitosan but is water soluble at physiological pH, allowing easier vaccine preparation and better overall performance.
- Nanoparticle surface display of antigens. Based on clever chitosan nanoparticle designs from the research literature, we redesigned B-cell epitopes to facilitate chitosan/HACC surface display of epitope peptides.
- Ii-key technology for enhancing the immune response. A small peptide tag called Ii-key was added to certain epitope sequences to amplify the T helper cell response, increasing the potency of both B-cell and T-cell components of the vaccine.
- Dendritic cell targeting. We introduced a peptide into our vaccine designs, adapted from recent publications showing enhanced antigen presentation and processing by targeting vaccine nanoparticles to professional antigen presenting dendritic cells.
- First RaDVaC conference. Unlocking Vaccines: Open-Source Vaccine Summit 2021 (all conference presentations available on YouTube here). Here are some highlights from the conference:
- Our chief scientist, Dr. Preston Estep, provided a comprehensive background on the RaDVaC project, and the urgent need for radical improvement in access to vaccines and the requisite knowledge and technologies for producing them. Dr. Estep highlighted valuable lessons that have been learned from open-source software, especially Linux, which has provided high performance with transparency and security.
- RaDVaC clinical trials liaison, Brian Delaney, and Professors Nir Eyal and Ben Hurlbut discussed the bioethics of public health research & vaccine deployment
- Member of EU Parliament Jutta Paulus, and policy researcher Milena Leybold joined us to provide insight from the policy perspective of vaccinology, bringing clarity to the obstacles that inhibit conventional public investment in more accessible vaccine infrastructure.
- Dr. Reza Mostofi presented data on the efficacy of RaDVaC vaccines in animals, and in human volunteers. His team found that the Gen 8 and Gen 9 vaccines show high efficacy as boosters, but much lower efficacy when used as a single dose in previously uninfected volunteers. These insights catalyzed improvements represented in our Gen 10 and later designs.
- RaDVaC member Dr. George Church provided insights from his decades of experience in pioneering (and self-experimenting with) highly leverageable biotechnologies, and the opportunity for those design & collaboration principles to have an impact on vaccinology and the future of global health.
- Dr. Craig Travis discussed the unique features and advantages of the mucosal immune system in combatting respiratory diseases, and more.
- Renowned economists Alex Tabarrok and Andrew Lo shared their views from the economic side of vaccinology. Dr. Tabarrok praised RaDVaC and acknowledged the challenges of accelerating the process of bringing a vaccine to market. Dr. Lo, a recognized worldwide authority on vaccine development and economics, presented and discussed historical data showing that vaccines are the most successful of all therapeutic classes, and contrasted their importance with poor returns on investment, underscoring the extreme undervaluation of vaccines within the framework of conventional bio/pharma development.
- Vaccine testing and trialing. Data on pre-Gen 10 vaccines guided us to many substantial improvements in later vaccine designs. Our vaccine platform is now better but the older data are not relevant to recent generations because the vaccines are different in every key feature. We are testing immune correlates of the most updated generations, and starting in January we will be testing newer generations that target omicron. A major challenge of a rapid deployment vaccine response to a rapidly evolving pathogen is the developmental timeline for maintaining effectiveness against the latest variant(s). This drives our ultimate goal to create an integrated plug-and-play vaccine design and trialing platform, allowing high-confidence of effectiveness upon initial vaccine deployment.
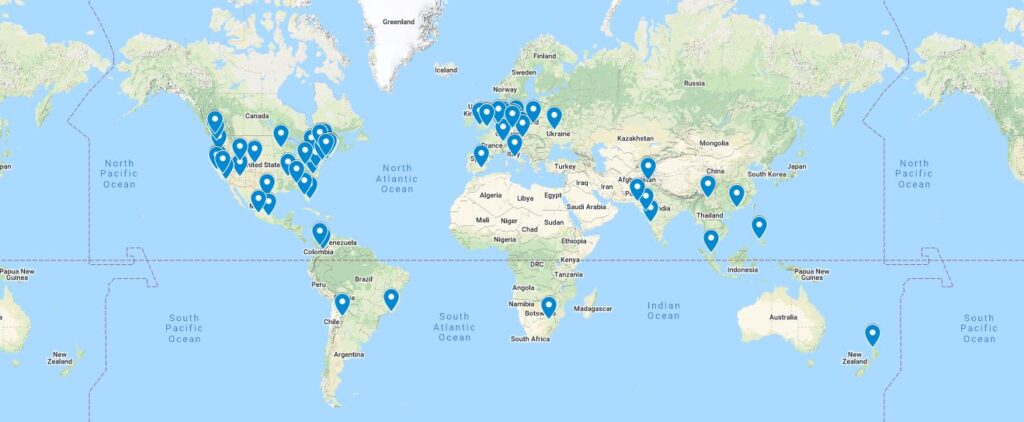
- Developers, developers, developers. Early this year we launched our Researchers Map, which helps scientists connect with potential collaborators, near and far. As of today the map has 80 entries from 23 countries. If you’re interested in working together on vaccine R&D, or know someone who is, drop a pin and tell everyone about your work and ambitions! The Researchers Map is cool and useful, but we need to grow the network and improve connectivity. If you are (or know) an experienced web or app developer who can help us improve our networking platform, please connect with us.
- Vaccine regulatory approvals, scaling, and commercialization. In order to grow the project to serve more people in need of vaccines, RaDVaC is participating in booting up startup companies, beginning with a newco in India.
- This newco is a Delaware C corporation with a public benefit subsidiary in India
- Dr. Estep is a co-founder and received a percentage of founding equity, and assigned ownership of those shares to RaDVaC.
- Newco is preparing to to raise a series A soon.
- Pre-clinical studies will be done in India, followed by clinical trials for regulatory approval.
- Other newcos can be booted up in other geographic jurisdictions. Let us know if you are interested.
- We hope that RaDVaC’s participation and equity stake will be a model for a new kind of venture philanthropy to achieve public benefit.
- This newco is a Delaware C corporation with a public benefit subsidiary in India
- Omicron variant vaccine design and white paper
- Although omicron is heavily mutated, the Gen 11 vaccine is largely unaffected because of our focus on conserved neutralizing epitopes. Nevertheless, we are taking the opportunity to improve our current vaccines generally, and to create a specific vaccine allowing us to track and analyze omicron-specific immunity.
- Gen 12 update coming soon. Omicron is taking over but delta remains in circulation, and generally appears to be more virulent than omicron. Therefore, the new white paper will provide vaccine designs to target both delta and omicron variants.
- This new design will allow for distinction of omicron and pre-omicron immunity.
- Recombinant antigen production. We have begun to explore the use of microorganisms to produce antigens, which have certain advantages over synthetic peptides. This research focus is our first step toward the possibility of a vaccine factory in a cell or test tube.
Looking ahead to 2022, we have exciting plans to continue the expansion of the RaDVaC project and network. If you are interested in working to expand vaccine access in times and places of greatest need, stay tuned and please connect with us! If you like what we do and want to make a financial contribution to support our free and open-source vaccinology work, you can contribute here.
Thank you again to all who have contributed to the growing success of RaDVaC. We look forward to doing great things for and with you in 2022.
Team RaDVaC
RaDVaC statement on the SARS-CoV-2 Omicron variant

Version 1.0
December 1, 2021
The Omicron variant of SARS-CoV-2 has over 50 mutations, with 32 mutations in the Spike protein alone1. Both of these numbers are far higher than for any previous variant of concern.
These mutations are so numerous that they are likely to impact transmissibility and virulence, but in ways that are currently impossible to predict. Nevertheless, the effects of certain mutations on specific current therapies and vaccines can be predicted with reasonable confidence.
Key observations on the high-impact mutations of Omicron variant:
- Omicron has 15 substitution mutations in the 200 amino acid receptor binding domain (RBD) of Spike.
- About half of these mutations are concentrated in the portion of RBD that interacts with human ACE2 receptor (referred to as the receptor binding motif).
- Four mutations are substitutions of amino acids that directly contact the ACE2 receptor: Q493R, Q498R, N501Y, Y505H
- For comparison, the Alpha, Beta, and Gamma variants each contain only one mutation in ACE2 contact residues (N501Y), and the Delta variant contains zero2.
- Omicron also has mutations at residues 452 and 484. Mutations at these positions in the Delta variant reduce the effectiveness of vaccines.
Based on structural evidence of interactions between neutralizing antibodies and the Spike protein, we make the following predictions:
- Effect on RBD-based vaccines: We predict that Omicron will substantially reduce effectiveness of RBD-based vaccines, some of which have been reported to be highly effective against previous variants.
- Effect on Spike-based vaccines: The effectiveness of Spike-based vaccines will be compromised because RBD portions of these vaccines will be substantially less effective. Portions of Spike involved in membrane fusion (which RaDVaC has included in multiple generations of published vaccine candidates) are not mutated in Omicron and will provide some protection, but overall protection will be reduced. These vaccines include BioNTech-Pfizer Comirnaty, Moderna Spikevax (mRNA-1273), Janssen/Johnson & Johnson COVID-19 vaccine, and AstraZeneca/Oxford ChAdOx1 (AZD1222).
- Effect on neutralizing antibody therapies:
- Regeneron monoclonal antibody cocktail REGN-COV2, consisting of antibodies REGN10933 and REGN10987.
- Two mutations in the Omicron RBD (K417N, E484K) have been shown to essentially abolish binding of antibody REGN109333,4 and three mutations in the RBM result in substitutions of amino acids contacted by REGN10987 (N440, G446, Q498)5. We therefore predict that the Regeneron cocktail will be largely ineffective against Omicron.
- Lilly monoclonal antibody cocktail consisting of antibodies bamlanivimab (LY-CoV555) and etesevimab (LY-CoV016).
- LY-CoV555 binding is reduced by mutations at K417N and E484, while LY-CoV016 binding is likely reduced by K417N6,7,8,9. We predict that the Lilly cocktail will be substantially less effective against Omicron.
- Vir-GlaxoSmithKline sotrovimab. Sotrovimab binds to the glycan of N343 and surrounding amino acids, and should be relatively unaffected by omicron mutations.
- Regeneron monoclonal antibody cocktail REGN-COV2, consisting of antibodies REGN10933 and REGN10987.
- Effect on RaDVaC vaccine candidates:
- Current RaDVaC peptide vaccine designs are largely resistant to the mutations of Omicron. This is in keeping with the strategy of RaDVaC vaccines to be more robust against variants.
- RaDVaC core B-cell epitope peptides target the fusion peptide. Only one optional B-cell epitope peptide targets the RBD. Full-length Spike-based vaccines also contain these epitopes, but RBD vaccines do not.
- Current RaDVaC designs target multiple immunodominant T-cell epitopes, none of which is affected by Omicron. Neither Spike-based vaccines nor RBD-based vaccines contain any of the verified, immunodominant cytotoxic T-lymphocyte epitopes. Such immunodominant T-cell epitopes are key to immunity that is durable and resistant to variation over the long term.
- Recent discoveries about SARS-CoV-2, in addition to Omicron, have suggested minor refinements to RaDVaC Gen 11. Therefore, RaDVaC will soon release the Gen 12 vaccine design.
- Current RaDVaC peptide vaccine designs are largely resistant to the mutations of Omicron. This is in keeping with the strategy of RaDVaC vaccines to be more robust against variants.
Summary: We predict the extensive variation of Omicron will reduce the effectiveness of Spike-based vaccines, and substantially reduce the effectiveness of RBD-based vaccines and antibody therapeutics (other than GSK sotrovimab), yet have little impact on RaDVaC vaccines.
RaDVaC remains committed to producing and distributing free, open-source, and up-to-date vaccine recipes and high-quality vaccine deployment tools, including a vaccine platform that is modular, easy to produce, rapid to adapt, and resilient to pathogen mutation. In addition to updates to our whitepapers, we are now increasing our investment in studies to validate and generalize the technologies we produce in order to create a plug-n-play vaccine platform that provides high confidence of effectiveness when used for the first time against a novel pathogen.
References:
- https://en.wikipedia.org/wiki/SARS-CoV-2_Omicron_variant#Mutations
- https://en.wikipedia.org/wiki/SARS-CoV-2_Delta_variant#Mutations
- https://www.science.org/doi/10.1126/science.abf9302
- https://www.nature.com/articles/s41586-021-03398-2
- https://science.sciencemag.org/content/369/6506/1010.abstract
- https://www.cell.com/cell-host-microbe/pdf/S1931-3128(21)00283-3.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8524965/
- https://www.nature.com/articles/s41598-021-99827-3
- https://www.sciencedirect.com/science/article/pii/S2666379121000719